Mahika Luthra (ESR#10) has just gotten her work published in ACS Catalysis!
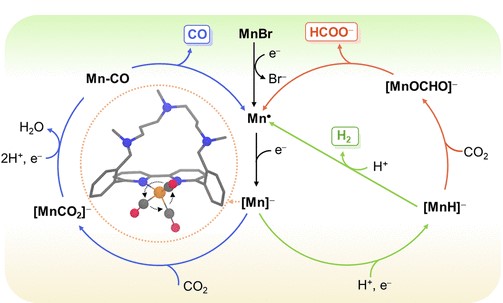
Abstract: Selective reduction of CO2 is an efficient solution for producing nonfossil-based chemical feedstocks and simultaneously alleviating the increasing atmospheric concentration of this greenhouse gas. With this aim, molecular electrocatalysts are being extensively studied, although selectivity remains an issue. In this work, a combined experimental–computational study explores how the molecular structure of Mn-based complexes determines the dominant product in the reduction of CO2 to HCOOH, CO, and H2. In contrast to previous Mn(bpy-R)(CO)3Br catalysts containing alkyl amines in the vicinity of the Br ligand, here, we report that bpy-based macrocycles locking these amines at the side opposite to the Br ligand change the product selectivity from HCOOH to H2. Ab initio molecular dynamics simulations of the active species showed that free rotation of the Mn(CO)3 moiety allows for the approach of the protonated amine to the reactive center yielding a Mn-hydride intermediate, which is the key in the formation of H2 and HCOOH. Additional studies with DFT methods showed that the macrocyclic moiety hinders the insertion of CO2 to the metal hydride favoring the formation of H2 over HCOOH. Further, our results suggest that the minor CO product observed experimentally is formed when CO2 adds to Mn on the side opposite to the amine ligand before protonation. These results show how product selectivity can be modulated by ligand design in Mn-based catalysts, providing atomistic details that can be leveraged in the development of a fully selective system.