Our Annual Report for 2022 is now published
Read all about us!
Our annual report gives an account of the research and activities of our network for all who wish to follow us up or learn more about us. 2022 was a year rich in travels, as our ESRs took full opportunity of the lifted travel restrictions to go to their secondments and all 15 of them were gathered for the very first time at our Annual Meeting in Rostock, Germany.
You can read the report here; it is also available from our Publications page.
Research article: Collaboration between AU and UiO!
Mahika Luthra (ESR#10) has just gotten her work published in ACS Catalysis!
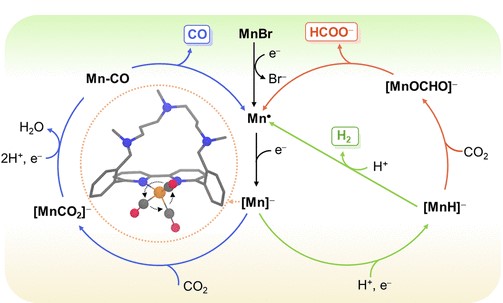
Abstract: Selective reduction of CO2 is an efficient solution for producing nonfossil-based chemical feedstocks and simultaneously alleviating the increasing atmospheric concentration of this greenhouse gas. With this aim, molecular electrocatalysts are being extensively studied, although selectivity remains an issue. In this work, a combined experimental–computational study explores how the molecular structure of Mn-based complexes determines the dominant product in the reduction of CO2 to HCOOH, CO, and H2. In contrast to previous Mn(bpy-R)(CO)3Br catalysts containing alkyl amines in the vicinity of the Br ligand, here, we report that bpy-based macrocycles locking these amines at the side opposite to the Br ligand change the product selectivity from HCOOH to H2. Ab initio molecular dynamics simulations of the active species showed that free rotation of the Mn(CO)3 moiety allows for the approach of the protonated amine to the reactive center yielding a Mn-hydride intermediate, which is the key in the formation of H2 and HCOOH. Additional studies with DFT methods showed that the macrocyclic moiety hinders the insertion of CO2 to the metal hydride favoring the formation of H2 over HCOOH. Further, our results suggest that the minor CO product observed experimentally is formed when CO2 adds to Mn on the side opposite to the amine ligand before protonation. These results show how product selectivity can be modulated by ligand design in Mn-based catalysts, providing atomistic details that can be leveraged in the development of a fully selective system.
Research article from AU and AstraZeneca!
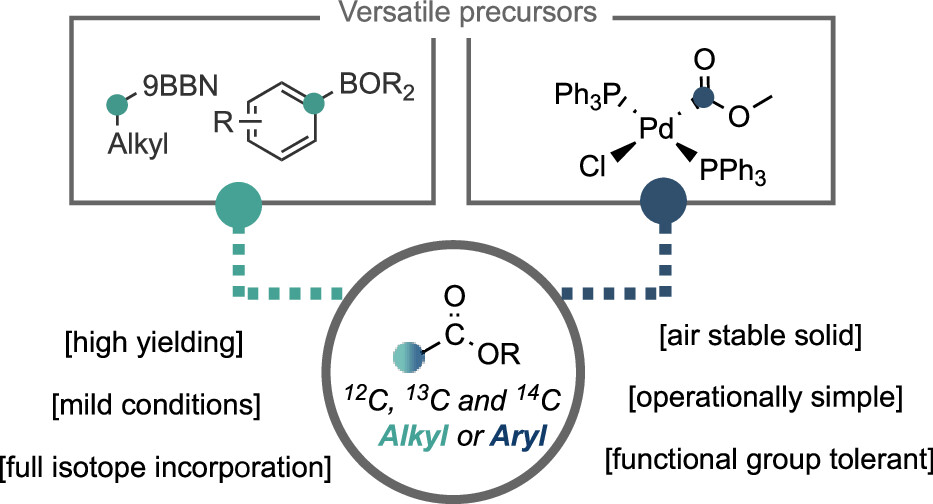
Abstract: Herein, we report a strategy for the formation of isotopically labeled carboxylic esters from boronic esters/acids using a readily accessible palladium carboxylate complex as an organometallic source of isotopically labeled functional groups. The reaction allows access to either unlabeled or full 13C- or 14C-isotopically labeled carboxylic esters, and the method is characterized by its operational simplicity, mild conditions, and general substrate scope. Our protocol is further extended to a carbon isotope replacement strategy, involving an initial decarbonylative borylation procedure. Such an approach allows access to isotopically labeled compounds directly from the unlabeled pharmaceutical, which can have implications for drug discovery programs.
Research article form AU.
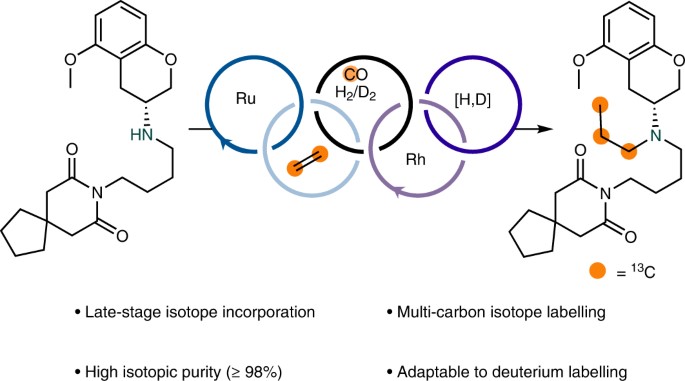
Abstract: Drug metabolism and pharmacokinetic studies play a crucial role in drug discovery and development programmes, assessing a lead drug candidate’s efficacy and safety profile. Quantitative bioanalytical assessment of analytes with mass spectrometry requires the use of stable carbon-13-labelled compounds with a molecular mass difference of ≥3 daltons. The incorporation of three or more carbon isotopes into drug candidates is not trivial, often requiring lengthy and costly syntheses. Here we report a dual catalytic strategy for the synthesis of multi-carbon-labelled isotopologues of active pharmaceutical ingredients. This approach uses isotopically labelled gas surrogates in a three-chamber reactor for sequential release of alkenes, carbon monoxide and hydrogen followed by low-pressure hydroformylation to generate multi-labelled alkyl aldehydes. The method’s utility has been demonstrated through the synthesis of multiple labelled N-alkyl bioactive compounds, site-selective carbon-13 and deuterium introduction and for triple-carbon labelling of small molecules combined with α-functionalization.
Did you think?
Today, inclusion in science is more important than ever. Studies around the world have shown that we still think differently about career opportunities for men and women, or for people from different races or societal minorities. How can we change that?
The exhibition “Did you think” shows photos of 14 researchers as children and as adults and their answers to questions related to their career path.
The aim of the exhibition is to influence the unconscious thoughts we all have when we look at girls and boys and think about their career possibilities. Our ESRs have selected their role models and we hope this serves as inspiration to pass on an important message of empowerment for future generations…let our kids know they can become whatever they want! Let us help them along the way!
Check it out here!

