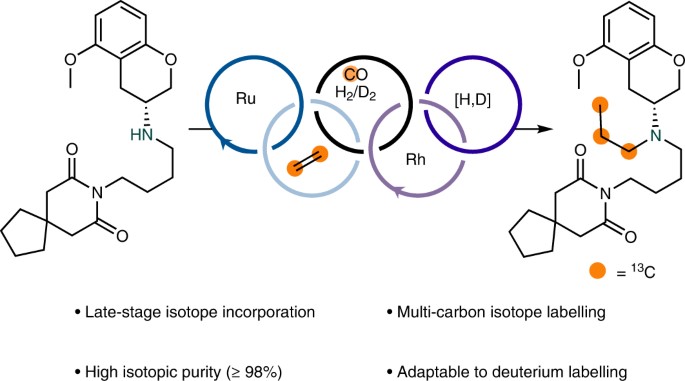
Abstract: Drug metabolism and pharmacokinetic studies play a crucial role in drug discovery and development programmes, assessing a lead drug candidate’s efficacy and safety profile. Quantitative bioanalytical assessment of analytes with mass spectrometry requires the use of stable carbon-13-labelled compounds with a molecular mass difference of ≥3 daltons. The incorporation of three or more carbon isotopes into drug candidates is not trivial, often requiring lengthy and costly syntheses. Here we report a dual catalytic strategy for the synthesis of multi-carbon-labelled isotopologues of active pharmaceutical ingredients. This approach uses isotopically labelled gas surrogates in a three-chamber reactor for sequential release of alkenes, carbon monoxide and hydrogen followed by low-pressure hydroformylation to generate multi-labelled alkyl aldehydes. The method’s utility has been demonstrated through the synthesis of multiple labelled N-alkyl bioactive compounds, site-selective carbon-13 and deuterium introduction and for triple-carbon labelling of small molecules combined with α-functionalization.

